What is Hydrolysis?
Hydrolysis is a chemical
reaction that involves the splitting of a chemical bond by adding water.
Hydrolysis literally means "water splitting." This process is
commonly used in organic chemistry and biochemistry, and it is an essential
part of many chemical and biological reactions.
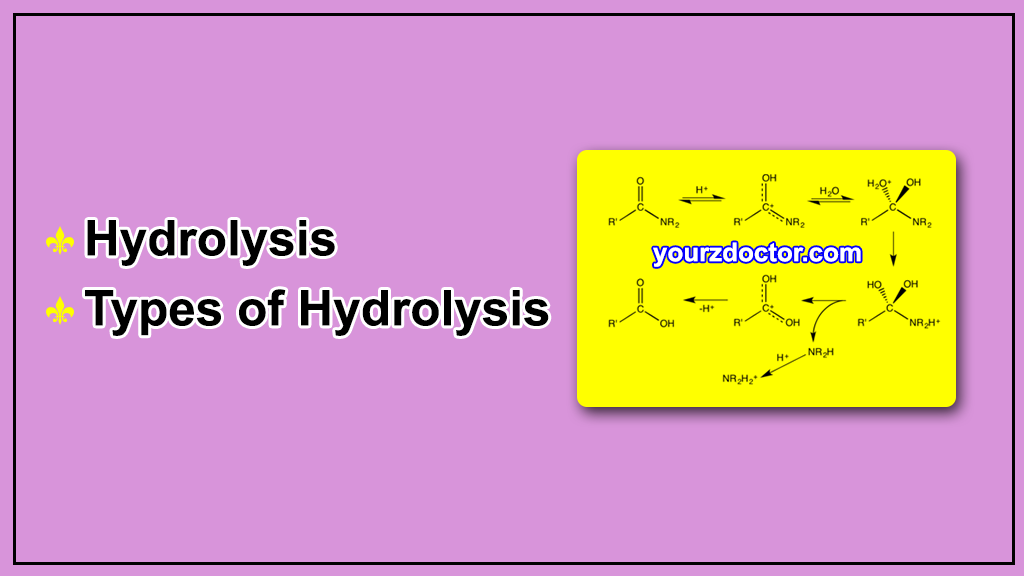
Hydrolysis involves the breaking of a bond between two atoms by the addition of a water molecule. The water molecule splits into a hydrogen ion (H+) and a hydroxide ion (OH-), and these ions then react with the atoms on either side of the bond being broken. The hydrogen ion (H+) attaches to one of the atoms, while the hydroxide ion (OH-) attaches to the other. This process results in the splitting of the molecule into two separate compounds.
Hydrolysis and Types of Hydrolysis
Types of Hydrolysis
There are
several types of hydrolysis, and each has its unique characteristics and
applications. The most common types of hydrolysis are discussed below:
1.
Acid Hydrolysis
Acid
hydrolysis is a chemical process that involves the breaking of a chemical bond
in a molecule by adding an acidic solution, such as sulfuric acid. In acid
hydrolysis, the hydrogen ion (H+) from the acid reacts with the molecule,
causing it to split into two separate compounds.
Acid
hydrolysis is commonly used in the production of biofuels and in the synthesis
of organic compounds. For example, in the production of ethanol from cellulose,
acid hydrolysis is used to break down the cellulose into glucose, which can
then be fermented into ethanol.
2.
Base Hydrolysis
Base
hydrolysis is a chemical process that involves the breaking of a chemical bond
in a molecule by adding a basic solution, such as sodium hydroxide. In base
hydrolysis, the hydroxide ion (OH-) from the base reacts with the molecule,
causing it to split into two separate compounds.
Base hydrolysis
is commonly used in the production of soaps and detergents. In this process, a
fat or oil is treated with a strong base, such as sodium hydroxide, to produce
a soap molecule and glycerol.
3.
Salt Hydrolysis
Salt
hydrolysis is a chemical process that involves the breaking of a chemical bond
in a molecule by adding a salt solution. In salt hydrolysis, the salt
dissociates into its component ions, which then react with the molecule,
causing it to split into two separate compounds.
Salt
hydrolysis is commonly used in the production of ceramics and glass. In this
process, a glass or ceramic material is treated with a salt solution to
dissolve the surface layer, producing a smoother surface.
4.
Enzymatic Hydrolysis
Enzymatic
hydrolysis is a chemical process that involves the breaking of a chemical bond
in a molecule by adding an enzyme. Enzymes are biological catalysts that speed
up chemical reactions in living organisms. Enzymatic
hydrolysis is used extensively in the digestion of food and in the breakdown of
complex biomolecules, such as proteins and carbohydrates.
Enzymatic
hydrolysis is also used in the production of biofuels and in the synthesis of
pharmaceuticals. For example, in the production of biodiesel from vegetable
oil, an enzyme called lipase is used to break down the oil into its component
fatty acids and glycerol.
5.
Photolytic Hydrolysis
Photolytic
hydrolysis is a type of hydrolysis that involves the use of light energy to
break a chemical bond in a molecule. This process is commonly used in the
degradation of pollutants in water, such as pesticides and herbicides. In
photolytic hydrolysis, a molecule absorbs light energy, which causes it to
split into two separate compounds.
Photolytic
hydrolysis is also used in the synthesis of organic compounds, such as in the
production of certain drugs and pharmaceuticals. This process can be used to selectively
break down specific chemical bonds in a molecule, leading to the production of
desired products.
Significance of Hydrolysis
Hydrolysis
is a critical process in many industrial, biological, and chemical
applications. Some of the significant applications of hydrolysis are discussed
below:
·
Production of Biofuels
Hydrolysis
is an essential process in the production of biofuels from renewable sources,
such as cellulose and vegetable oil. In the production of ethanol from
cellulose, acid hydrolysis is used to break down the cellulose into glucose,
which can then be fermented into ethanol. Similarly, in the production of
biodiesel from vegetable oil, enzymatic hydrolysis is used to break down the
oil into its component fatty acids and glycerol.
·
Synthesis of Organic
Compounds
Hydrolysis
is used extensively in the synthesis of organic compounds, such as in the
production of pharmaceuticals and fine chemicals. Acid hydrolysis and base
hydrolysis are commonly used in the production of organic compounds, as they
can selectively break down specific chemical bonds in a molecule.
·
Degradation of Pollutants
Hydrolysis
is used in the degradation of pollutants in water and soil, such as pesticides
and herbicides. Photolytic hydrolysis is commonly used in the degradation of
these pollutants, as it can selectively break down specific chemical bonds in a
molecule.
·
Digestion of Food
Hydrolysis
is a critical process in the digestion of food. Enzymatic hydrolysis is used to
break down complex biomolecules, such as proteins and carbohydrates, into their
component parts, which can then be absorbed by the body.
Protection of Drugs Against Hydrolysis
One of the
critical factors that affect the efficacy of drugs is their stability in
various environments, including in the presence of water. Hydrolysis is a
common degradation pathway for drugs in aqueous environments, and it can lead
to the loss of drug activity or the formation of toxic
by-products. As a result, the protection of drugs against hydrolysis is a
crucial aspect of drug development.
There are
several methods used to protect drugs against hydrolysis, including:
1)
Prodrug Design
Prodrug
design is a strategy used to improve the pharmacokinetics and pharmacodynamics
of drugs. Prodrugs are biologically inactive compounds that are converted into
active drugs in the body by metabolic or chemical reactions. Prodrug design can
be used to protect drugs against hydrolysis by modifying the chemical structure
of the drug molecule, making it less susceptible to hydrolysis.
For example,
the antiviral drug valacyclovir is a prodrug of acyclovir, which is used to
treat herpes virus infections. Valacyclovir is more stable in aqueous environments
than acyclovir, making it a more effective treatment option.
2)
Polymer Coating
Polymer
coatings can be used to protect drugs against hydrolysis by creating a physical
barrier between the drug molecule and the surrounding environment. The polymer
coating can be designed to be selectively permeable, allowing the drug molecule
to be released slowly over time. This approach is commonly used in the
development of sustained-release drug formulations.
3)
pH Adjustment
Hydrolysis
rates can be influenced by the pH of the environment in which the reaction
occurs. By adjusting the pH, it is possible to slow down or prevent hydrolysis
of a drug molecule. For example, the anticancer drug carboplatin is less
susceptible to hydrolysis at lower pH values, making it more stable in acidic
environments.
4)
Chemical Modification
Chemical
modification of drug molecules can be used to protect them against hydrolysis.
Chemical modification involves the addition of functional groups to the drug
molecule that are less susceptible to hydrolysis than the original chemical
bonds. For example, the addition of a methyl group to the antibiotic penicillin
can protect it from hydrolysis by bacterial enzymes.
Conclusion
Hydrolysis
is a significant degradation pathway for drugs in aqueous environments. The
protection of drugs against hydrolysis is essential for the development of safe
and effective treatments. Prodrug design, polymer coatings, pH adjustment, and
chemical modification are some of the strategies used to protect drugs against
hydrolysis. By understanding the mechanisms of hydrolysis and the methods used
to protect drugs against it, scientists and engineers can develop more stable
and effective drugs.
Related Tags,
hydrolysis,salt hydrolysis,hydrolysis and dehydration synthesis,hydrolysis of salt,hydrolysis of salts,hydrolysis of amides,hydrolysis of water,ph of salt hydrolysis,ester hydrolysis,what is hydrolysis,types of hydrolysis,most common types of hydrolysis,types of salt hydrolysis - salt reacts with water.,salt hydrolysis iit jee,dehydration synthesis and hydrolysis,salt hydrolysis questions,hydrolysis of carbohydrates,hydrolysis of a salt





0 Comments