In chemistry, a buffer is a solution that resists changes in pH when an acid or base is added to it. Buffers are essential in many chemical reactions and biological processes, including blood pH regulation, digestion, and enzyme activity. In this blog post, we will discuss buffers and the buffer equation, which is an essential tool for calculating the pH of a buffer solution.
What is a Buffer?
A buffer
is a solution that can resist changes in pH when an acid or base is added to
it. This means that when a small amount of acid or base is added to a buffer
solution, the pH of the solution does not change significantly. Buffers are
important in many chemical reactions and biological processes because they help
maintain a stable pH environment.
Buffers can
be made from a weak acid and its conjugate base or a weak base and its
conjugate acid. When a weak acid is added to water, it partially dissociates
into its conjugate base and hydrogen ions (H+). For example, acetic acid
(CH3COOH) partially dissociates into acetate ions (CH3COO-) and hydrogen ions
(H+). The equilibrium constant for this reaction is given by the equation:
Buffer and Buffer Equation
CH3COOH + H2O ⇌ CH3COO- + H3O+
The
equilibrium constant for this reaction is called the acid dissociation constant
(Ka), and it is a measure of the strength of the acid. The larger the Ka value,
the stronger the acid. For acetic acid, the Ka value is 1.8 × 10^-5, which
means it is a weak acid.
When a
strong acid, such as hydrochloric acid (HCl), is added to a solution of acetic
acid and acetate ions, the hydrogen ions from the strong acid react with the
acetate ions to form acetic acid.
This reaction can be written as follows:
HCl + CH3COO- ⇌ CH3COOH + Cl-
This
reaction consumes the hydrogen ions from the strong acid, which prevents the pH
of the solution from decreasing significantly. Similarly, when a strong base,
such as sodium hydroxide (NaOH), is added to the buffer solution, it reacts
with the acetic acid to form acetate ions and water. This reaction can be
written as follows:
NaOH + CH3COOH ⇌ CH3COO- + H2O
This
reaction consumes the acetic acid
from the buffer solution, which prevents the pH of the solution from increasing
significantly.
Buffer Capacity:
The buffer
capacity is a measure of the ability of a buffer solution to resist changes in
pH. It depends on the concentrations of the weak acid and its conjugate base in
the buffer solution. The buffer capacity is maximum when the concentrations of
the weak acid and its conjugate base are equal.
The buffer
capacity is also affected by the pH of the buffer solution. Buffers have
maximum buffer
capacity at a pH equal to the pKa of the weak acid. At this pH, the
concentrations of the weak acid and its conjugate base are equal, which
maximizes the buffer capacity.
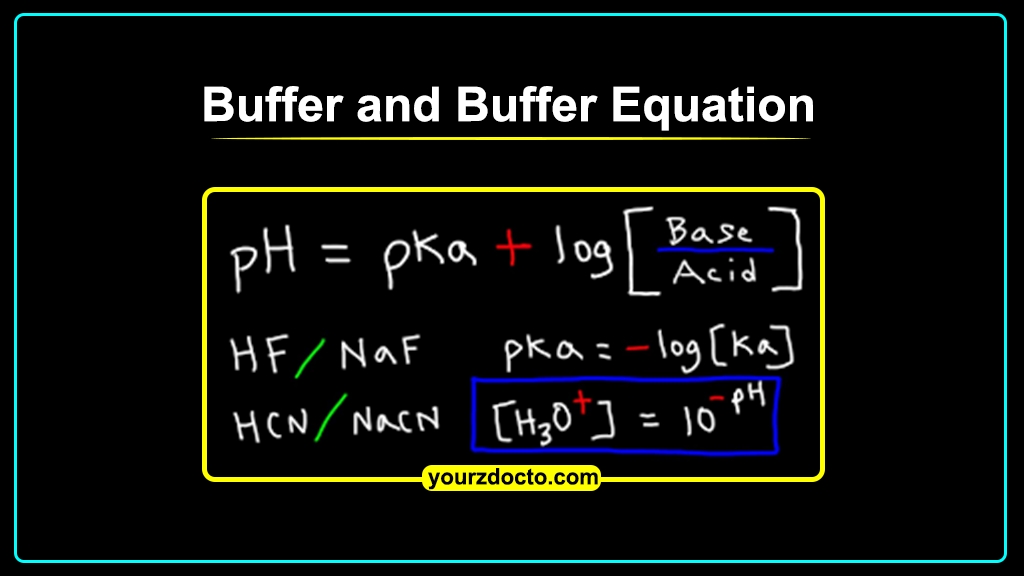
Buffer Equation:
The buffer
equation is an important tool for calculating the pH of a buffer solution.
It is based on the Henderson-Hasselbalch
equation, which relates the pH of a buffer solution to the pKa of the weak
acid and the concentrations of the weak acid and its conjugate base.
The
Henderson-Hasselbalch equation is given by the following equation:
pH = pKa
+ log([conjugate base]/[weak acid])
Where pH is
the pH of the buffer solution, pKa is the acid dissociation constant of the
weak acid, [conjugate base] is the concentration of the conjugate base, and
[weak acid] is the concentration of the weak acid in the buffer solution.
The buffer
equation can be used to calculate the pH of a buffer solution when the
concentrations of the weak acid and its conjugate base are known. To use the buffer
equation, we first need to determine the pKa of the weak acid. This can be
found in a table of acid dissociation constants for common weak acids.
Once we know
the pKa, we can calculate the ratio of conjugate base to weak acid using the
following equation:
[conjugate
base]/[weak acid] = 10^(pH-pKa)
Using this
ratio and the concentration of either the weak acid or the conjugate base, we
can calculate the concentration of the other species. For example, if we know
the concentration of the weak acid and the ratio of conjugate base to weak
acid, we can calculate the concentration of the conjugate base using the
following equation:
[conjugate
base] = [weak acid] × [conjugate base]/[weak acid]
Once we have
determined the concentrations of the weak acid and its conjugate base, we can
substitute them into the Henderson-Hasselbalch equation to calculate the pH of
the buffer solution.
Example:
Let's
consider an example of a buffer solution made from acetic acid (CH3COOH) and
its conjugate base, acetate ions (CH3COO-). The pKa of acetic acid is 4.76. We
have a buffer solution containing 0.1 M of acetic acid and 0.2 M of acetate
ions. What is the pH of the buffer solution?
First, we
need to determine the ratio of conjugate base to weak acid using the following
equation:
[conjugate
base]/[weak acid] = 10^(pH-pKa)
Substituting
the values, we get:
0.2/0.1 =
10^(pH-4.76)
2 =
10^(pH-4.76)
Taking
the logarithm of both sides, we get:
log(2) =
pH-4.76
pH = log(2)
+ 4.76
pH = 4.88
Therefore,
the pH of the buffer solution is 4.88.
Conclusion:
Buffers are
important in many chemical reactions and biological processes because they help
maintain a stable pH environment. Buffers can be made from a weak acid and its
conjugate base or a weak base and its conjugate acid. The buffer capacity is a
measure of the ability of a buffer solution to resist changes in pH, and it
depends on the concentrations of the weak acid and its conjugate base in the
buffer solution. The buffer equation is an essential tool for calculating the
pH of a buffer solution, and it is based on the Henderson-Hasselbalch equation,
which relates the pH of a buffer solution to the pKa of the weak acid and the
concentrations of the weak acid and its conjugate base. By using the buffer
equation, we can determine the pH of a buffer solution when the concentrations
of the weak acid and its conjugate base are known.
Related Tags,
buffer solution,buffer equation,buffers,buffer action,buffer,buffer solution (chemical compound),buffer capacity,buffer solution and buffer action,buffer calculations,buffer equation derivation,buffer solution preparation,buffer equations,buffer equation for ph,handerson equation for buffer solution,application of buffer solution,buffer equation depth of biology,ph buffer and isotonic solution,buffer solution ph calculations,buffer solution and its types





0 Comments